Abstract
Introduction:
Nilotinib is a second-generation BCR-ABL1 tyrosine kinase inhibitor (TKI) widely used for the treatment of Philadelphia chromosome positive chronic myeloid leukemia (CML). ENESTnd, which compared two doses of nilotinib to imatinib for patients with newly diagnosed CML, demonstrated superior rates of major molecular responses (MMRs) at 12 months in patients treated with nilotinib. However, a relative increase in cardiovascular events (CVE) was observed, including ischemic heart disease, stroke, and/or peripheral artery disease among nilotinib-treated patients. Notably, at a median 5-year follow up, 7.5% of patients on nilotinib 300mg twice daily experienced a CVE (Hochaus, Leukemia, 2016) and by 10-years of follow up, approximately 20% of patients treated with nilotinib developed a CVE (Kantarjian, Leukemia, 2021). The baseline Framingham general cardiovascular risk scores and total dose exposure were predictive of patients' risk of developing CVE.
In the province of Quebec, Canada, the GQR LMC-NMP (Groupe Québécois de recherche en leucémie myéloide chronique et néoplasie myéloproliférative) has maintained a registry of nearly all patients with CML since 2011. Using this 900+-patient registry, we examined molecular responses and the rate of CVE among patients receiving nilotinib as frontline therapy for CML, to determine if they were similar to those observed in ENESTnd.
Methods:
We identified all patients with chronic phase CML in the registry who were initiated on nilotinib for chronic phase CML. Baseline patient characteristics included were age, sex, Sokal index, baseline comorbidities, molecular response, cardiovascular complications, duration of exposure to nilotinib, dose adjustment, dose discontinuation and CVE on therapy.
Results:
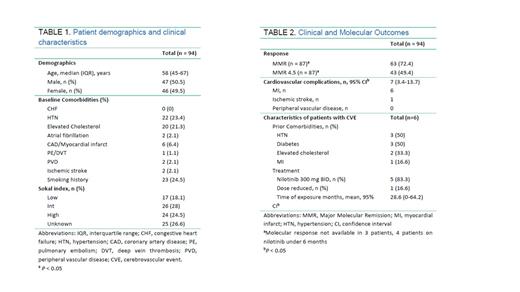
In total, 94 patients received nilotinib as frontline treatment starting in 2008 (Table 1). The median age of the population was 58 years, 50.5% were male. The most common cardiovascular comorbidity prior to nilotinib treatment was hypertension (23.4%) followed by elevated cholesterol (21.3%), and prior CVE (12%). Seventeen patients had a low Sokal index, 26 intermediate and 24 high, 25 missing. Sixty-five patients received nilotinib at a total of 600 mg per day, 29 patients had a dose reduction at some point in their treatment and 18 patients were on either 300 or 400 mg daily. The median follow-up time was 67.0 months (range 0.5 to 159), and median nilotinib exposure time was 38.5 months (range 0.5 to 144). Forty-six patients (48.9%) discontinued nilotinib, 6 for a treatment free remission (TFR). While on nilotinib, 63 patients achieved MMR (72.4%) and 43 achieved MR 4.5 (49.6%) by 5 years of treatment, with 52 (55.3%) and 22 (23.4%) patients achieving MMR or MMR4.5 within a year, respectively.
There was a total of 7 CVE in 6 patients: six myocardial infarctions and one ischemic stroke (Table 2). No patients developed peripheral artery disease. The mean time of exposure to nilotinib in these 6 patients was 28.6 months. Two patients had a high Sokal index, 3 intermediate and 1 low. One patient received dose-reduced nilotinib. These patients were co-morbid with diabetes (3), hypertension (3), elevated cholesterol (1) and one had a myocardial infarct prior to starting nilotinib, two patients did not have any risk factors. Only one of these patients has remained on nilotinib. All patients with a CVE were alive at the time of this report.
Conclusion:
We present real world data on the efficacy and safety of nilotinib in the frontline treatment of CML. Our data indicate that molecular responses and toxicities are similar to the ENESTnd trial. Of the 94 treated patients, the rate of MMR and CVE were 72% and 7.4% at 5.5 years of follow up, respectively, which is similar to that observed in ENESTnd, where 77% of patients experienced MMR and 7.5% CVE at this timepoint (Hochaus, Leukemia, 2016). All but one patient with a CVE in our study continued nilotinib, suggesting a reluctance among physicians to continue in the face of CVE. While there were 11 patients with prior CVE, only one of these developed a CVE on nilotinib. Current GQR-LMC CML management guidelines recommend estimation of Framingham score and management of cardiovascular risk factors for all patients with CML. Future follow up of these registry data may demonstrate a decrease in the rate of CVE among nilotinib treated patients, reflecting local adherence to these guidelines.
Busque: Novartis: Consultancy. Assouline: Eli Lilly: Research Funding; Jewish General Hospital, Montreal, Quebec: Current Employment; Novartis: Honoraria, Research Funding; Amgen: Current equity holder in publicly-traded company, Research Funding; Gilead: Speakers Bureau; Johnson&Johnson: Current equity holder in publicly-traded company; Roche/Genentech: Research Funding; Takeda: Research Funding; BeiGene: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; AstraZeneca: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal